Restiex® is a delayed-swelling hydrogel and is available for experimentation but not for clinical use as a tissue expander. Restiex is protected under patent: Park, Kinam, Clark Tobias Barco, Haesun Park, Yourong Fu, and John Solomon Garner. "Novel hydrogel tissue expanders." U.S. Patent Application 15/304,495, filed February 9, 2017. This technology is available for licensing. Additionally, samples for use in non-clinical research applications can be procured under a Materials Transfer Agreement. Contact us for more info.
Restiex®
Re-Shapable Tissue Expanding Hydrogel
Restiex® Presentation
This PowerPoint was used in our Controlled Release Society (CRS) 2011 presentation.![]()
Product Information
Most current tissue expanders are comprised of a silicon rubber balloon through which saline is injected causing the balloon to inflate stretching the tissue. These expanders must be injected repeatedly over the course of several weeks to months in order to stretch the tissue to the desired size. Due to these multiple injections and these materials being non-biocompatible the incidence of infection with this procedure is fairly high (an estimated 2-15%). Other "self-filling" expanders are made with a silicone shell which controls the expansion rate and can only be used as the purchased size and shape without being modified prior to implantation. To overcome these problems the PolySciTech® division of Akina has been researching an in-house proprietary hydrogel material that has inherent properties controlling its expansion without the need for an external membrane.
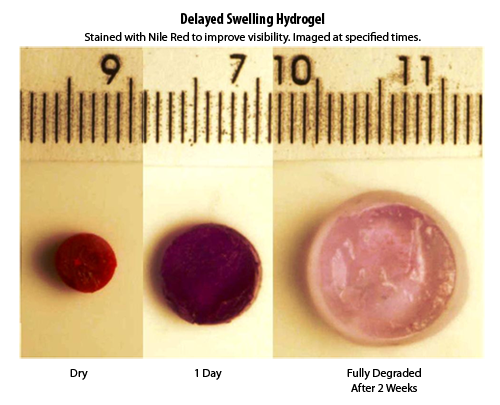
An example hydrogel swelling in a delayed fashion (dyed red for visibility).
The objective of current tissue expanding hydrogels research at PolySciTech, Inc. is to develop a novel hydrogel-based tissue expander that has delayed expansion with the capacity to be reshaped by the surgeon at the time of implantation. This is accomplished by chemically conjugating "delay" components with "swelling" components so that as the hydrogel is exposed to moisture its expansion is in a slow and delayed manner allowing for the tissue to be expanded without damage or necrosis.
By altering the composition of the "swelling" and "delay" components these hydrogels can have their swelling properties altered.
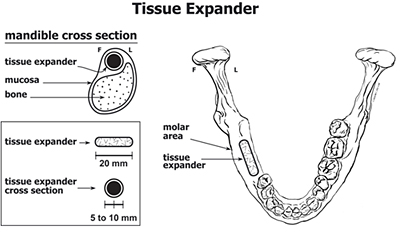
Hydrogel usage in a dental application.

A hydrogel expander normal and being
flexed between fingers. Note its elasticity.
Also, judicious selection of these ingredients creates an expander that is very flexible and soft while it is dry allowing it to be cut by the surgeon at the time of implantation allowing voids of any size and shape to be custom filled.
These hydrogels can serve as tissue expanders by having their delay time preprogrammed to allow the initial incision to heal prior to expansion. Phase I research performed at PolySciTech®, supported by NIH small business innovative research grant, has established that these hydrogels can be made that fit the requirements necessary for a surgical device. PolySciTech has been awarded a phase II research grant by the NIH and is currently determining safety and efficacy of these devices for use in dental applications. These materials have shown promise when used in animal models. We are interested in partnering with another company which has the manufacturing capabilities to generate these materials at medical grade quality. Please contact us to discuss this opportunity further.
Publications
Garner, John, Darrell D. Davidson, Daria Barwinska, George J. Eckert, Sunil S. Tholpady, Kinam Park, and Clark T. Barco. “Reshapeable hydrogel tissue expander for ridge augmentation: Results of a series of successive insertions at the same intraoral site.” Journal of Periodontology (2019).
Garner, John, Darrel Davidson, George J. Eckert, Clark T. Barco, Haesun Park, and Kinam Park. “Reshapable polymeric hydrogel for controlled soft-tissue expansion: in vitro and in vivo evaluation.” Journal of Controlled Release 262 (2017): 201-211.
Kun Young Yuk, Ye-Tae Kim, Su Jin Im, John Garner, Yourong Fu, Kinam Park, Jeong-Sook Park, Kang Moo Huh “Preparation and Characterization of Biodegradable Hydrogels for Tissue Expander Application” Polymer(Korea), Vol.34, No.3, 253-260, 2010.
Book Chapter: T.H. Tran, J. Garner, Y. Fu, K. Park, K.M. Huh “Biodegradable Elastic Hydrogels for Tissue Expander Application” in Handbook of Biodegradable Polymers, Wiley-VCH Verlag GmbH & Co. KGaA, pp. 217-236
Daria Barwinska, PhD, John Garner, BS, Darrell D. Davidson, MD, PhD, Todd G. Cook, AAS, George J. Eckert, MAS, Sunil S. Tholpady, MD, PhD, Keith L. March, MD, PhD, Kinam Park, PhD, Clark T. Barco, DDS, MS. “Mucosal Perfusion Preservation by a Novel Shapeable Tissue Expander for Oral Reconstruction.” PRS Global Open (2017)
Huh, Kang, You Choi, Jae Park, and Kinam Park. "Readily shapeable xerogels having controllably delayed swelling properties." U.S. Patent Application 11/495,153, filed February 8, 2007.
Park, Kinam, Clark Tobias Barco, Haesun Park, Yourong Fu, and John Solomon Garner. "Novel hydrogel tissue expanders." U.S. Patent Application 15/304,495, filed February 9, 2017.
 Akinalytics
Akinalytics Polymer Blog
Polymer Blog






