Stem-Cell Differentiation

Introduction
Generally, all cells in the human body are of a specific cell type. This could be bone cells, muscle cells, liver cells, kidney cells, lung cells, neural cells, and so forth with each type of cell associated with its specific organ and location. There are, however, in certain locations stem-cells. Stem-cells are undifferentiated cells (e.g. no specific type of cell) which can turn into specific types of cells based on their environmental conditions. This process is refered to as ‘differentiation’ in which the cell converts from being a stem-cell into a specific type of cell (neural, muscle, fat, etc.). It is important to note that the conditions which induce this are already programmed into the stem cell’s DNA. A powerful application of this process in terms of human technology is developing techniques to ‘convert’ stem cells into specific cell types. This could potentially be used, medically, for applications such as tissue or organ replacement. This could allow for a patient’s damaged organs or tissue to be replaced easily without needing to remove them from an organ donor or from another location in the patient’s body.
Many of the environmental conditions are chemical in nature, including various signaling molecules. This, classically, includes the use of dexamethasone, ascorbic acid and β-glycerophosphate to induce osteogenic differentiation forcing stem cells to turn into osteoblasts (bone-forming cells) and other differentiation factors. These are not the only factors, however.
Recently, researchers have found that not only chemical factors but also mechanical factors play a role in stem-cell differentiation. One factor is mechanical stress applied to the cells due to the surrounding matrix they grow in (Buxboim, Amnon, and Dennis E. Discher. "Stem cells feel the difference." Nature methods 7, no. 9 (2010): 695, Tenney, Rebeca M., and Dennis E. Discher. "Stem cells, microenvironment mechanics, and growth factor activation." Current opinion in cell biology 21, no. 5 (2009): 630-635.)
For example, researchers found that the gradients of stress are critical in terms of how cells either differentiate into either osteogenic (bone) or adipocytes (Fat tissue). This also occurs in a spatial matter due to cell traction. (Ruiz, Sami Alom, and Christopher S. Chen. "Emergence of patterned stem cell differentiation within multicellular structures." Stem cells 26.11 (2008): 2921-2927.) In fact, specific values for the elastic moduli that encourages differentiation towards specific cell-types have been identified and are listed in the table below.
Table 1. Mechanical properties and Stem-Cell differentiation patterns.
| Organ/Tissue Type | Elastic Modulus | Reference |
|---|---|---|
Neural cells (Brain/nerves) |
0.1-1 kPa |
|
Adipogenic (Fat cells) |
1-10 kPa |
|
Muscle |
8-17 kPa |
|
Bone/Osteogenesis |
17 kPa |
|
Bone/Osteogenesis |
34 kPa |
|
Bone/Osteogenesis |
11-30 kPa |
Method
Each polymer was dissolved first in cold (~4° C) water with shaking at indicated concentration (% w/v) concentration in media. Dissolution was achieved by shaking cold for at least overnight in complete cell-growth media. Rheology was performed on an AR550 (TA instruments) with 60 mm 2 degree cone. Viscosity of solution at 0.1 (sec-1) and 5C was measured (1 minute peak hold 5 second test intervals). Rheology performed by oscillating at constant 6.283 rad/s, 0.1% strain, in increments of 1° C ranging from 5-45° C with 2 minutes of temperature equilibration at each point.
Results
Table 2. Rheological properties of indicated 3DCellMaker Gels at each concentration.
| Solution | G’ (37 °C) (Pa) | G” (37 °C) (Pa) | Volume (media) to add in a 5 ml kit |
|---|---|---|---|
4% w/v A3DH |
0.1044 Pa |
0.329 Pa |
6.25 ml |
5% w/v A3DH |
27.53 Pa |
4.664 Pa |
5.00 ml |
9% w/v A3DC |
69.15 |
21.26 Pa |
5.55 ml |
10% w/v A3DC |
1067 Pa |
295.4 Pa |
5.00 ml |
11% w/v A3DC |
2153 Pa |
662.1 Pa |
4.55 ml |
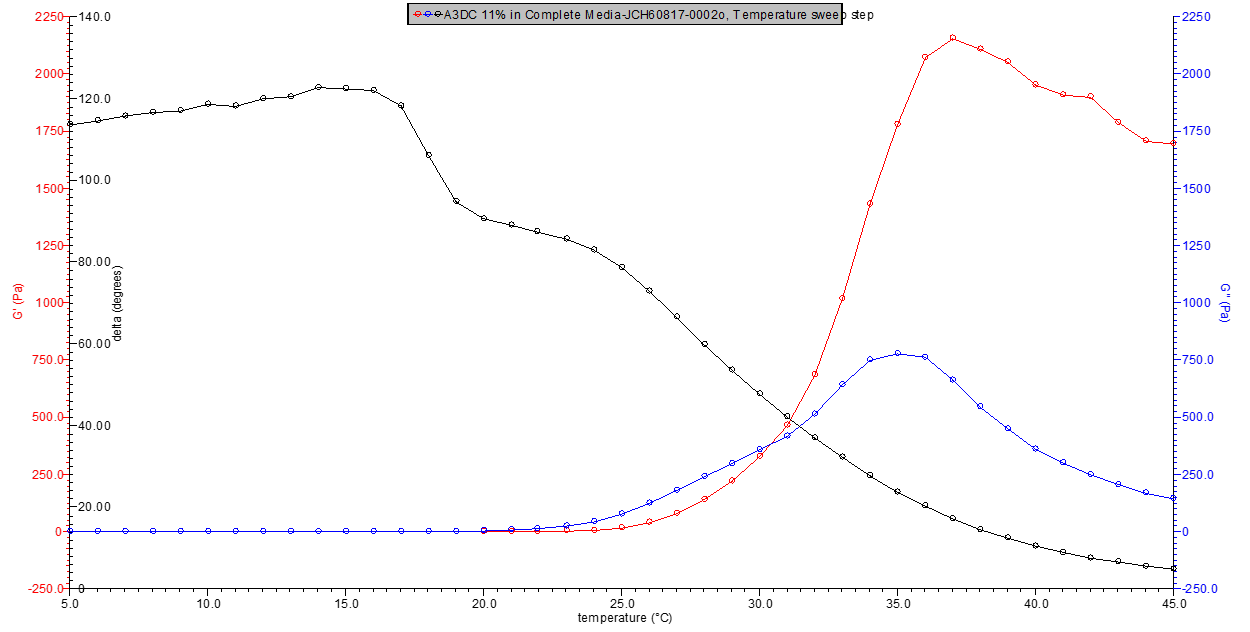
Figures 1-5 below show the rheological profile for each 3DCellMaker. For the commercially provided kits, the amounts included in each are preselected as A3DH-5ml = 0.25 grams, A3DH-10 ml = 0.5 grams, A3DC-5 ml = 0.5 grams, and A3DC-10 ml = 1 gram. By varying the amount of media added, various concentrations can be made in order to adjust the firmness of the resultant thermogel.
Figure 1. 4% w/v A3DH in media rheology profile.
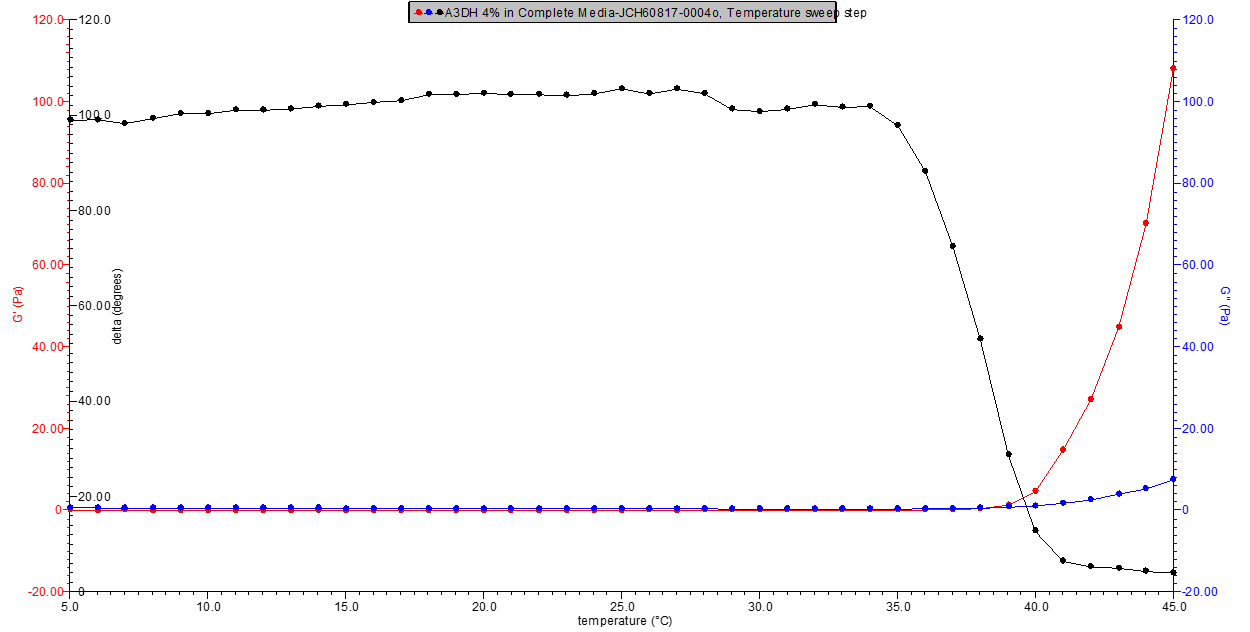
Figure 2. 5% w/v A3DH in media rheology profile.
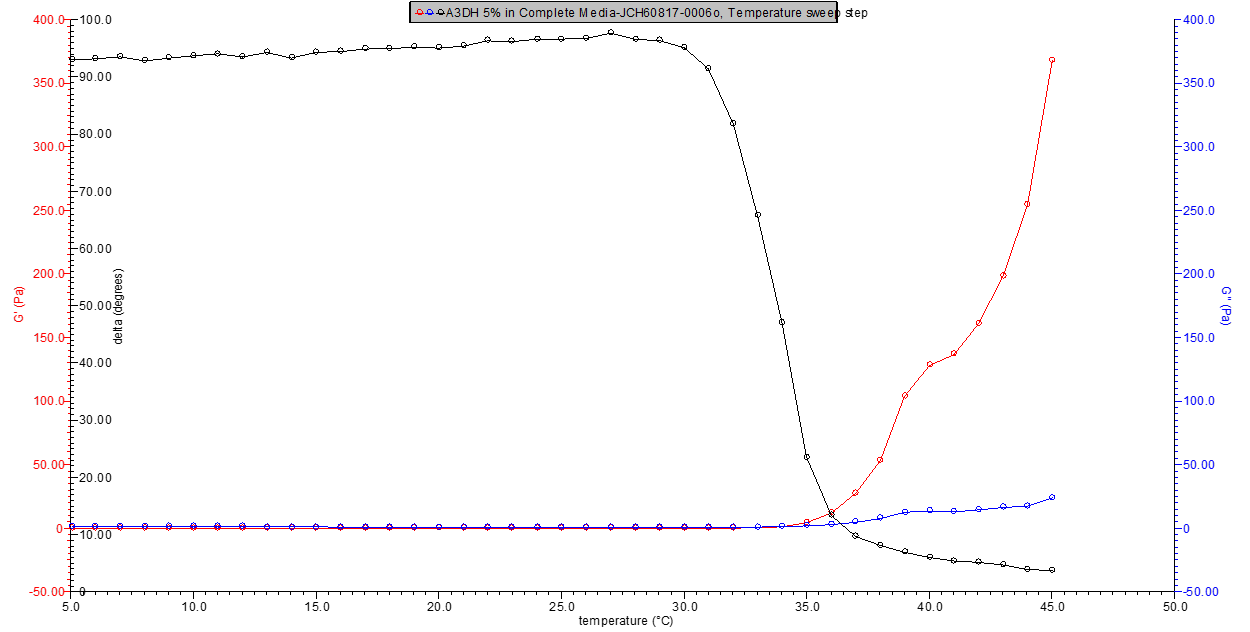
Figure 3. 9% w/v A3DC in media rheology profile.
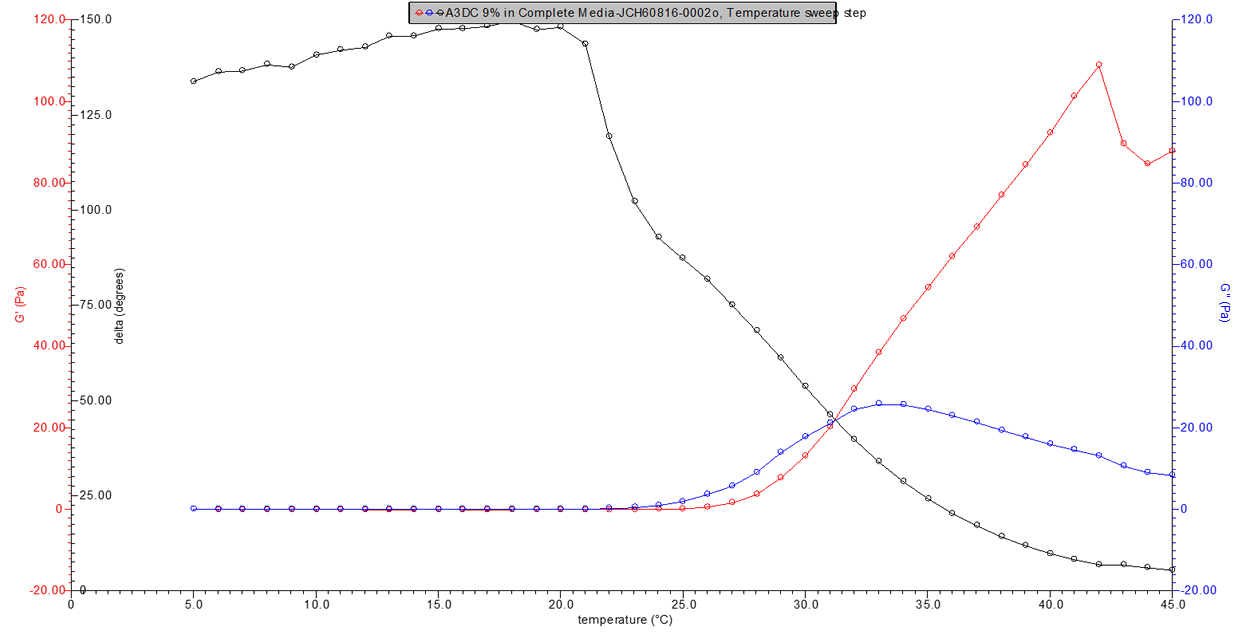
Figure 4. 10% w/v A3DC in media rheology profile.
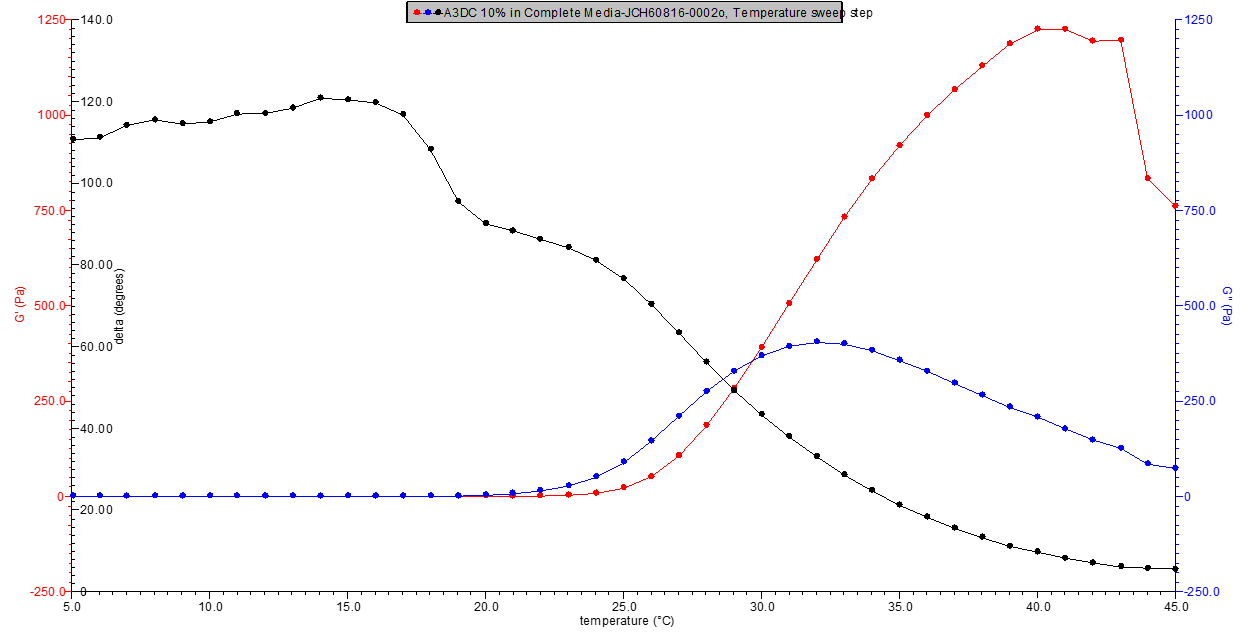
Figure 5. 11% w/v A3DC in media rheology profile.
Conclusion
By varying the concentration of 3DCellMaker in cell-growth media, a range of mechancial firmness properties can be developed and this may find applications towards stem-cell differentiation applications.
 Akinalytics
Akinalytics Polymer Blog
Polymer Blog






